- ABOUT US
- RESEARCH
- INNOVATION
- NEWS
- Suffering from depression? Hong Kong team can help with AI and by looking into your eyes, 2 Sep 2024
- BOC Life 9th Golden Age Expo & Summit, 2-4 August 2024
- 2024年創新科技獎項嘉許禮 – 15 July 2024
- CCRB developed a computational model to accurately forecast viral genetic evolution, 2 May 2024
- CCRB won two Gold Medals and a Special Award in the 49th International Exhibition of Inventions Geneva, 16-20 April 2024
- 杨浦创业之星大赛「创业之星」奖, 22 March 2024
- CUHK establishes Global Sports and Wellness Innovation Centre to develop ARIA for sports industry, 20 Feb 2024
- Transforming health-tech to sports-tech through University-Industry collaboration, 16 February 2024
- International Invention Fair in the Middle East (4-7 Feb 2024)
- International Nurses Day – Free ARIA test for all nurses, 12-31 May 2023
- CUHK develops an efficient approach to estimate the risk of heart disease in people living with HIV, 23 March 2023
- PARTNERS
- LINKS
- ARCHIVED
- Asia Summit on Global Health, 10 Nov 2022
- Bioinformatics method to predict COVID-19 vaccine effectiveness – 14 July 2022
- International ICT Expo and HK International Medical and Healthcare Fair, 27-30 Oct 2021
- CUHK Innovation and Entrepreneur Day, 23 September 2021
- CUHK Develops a Computational Platform to Predict Vaccine Effectiveness by Virus Genome, 7 May 2021
- Reuters Report on 15 March 2021 – HK scientist develops retinal scan technology to identify early childhood autism
- Automatic Retinal Image Analysis (ARIA) for Autism Spectrum Disorder, 4 Jan 2021
- Youth AI Health Ambassador Training and Outreach Program for Brain Health 2020-2021
- Outstanding Social Capital Partnership Award, 15 Dec 2020
- New AI technology to enhance flu vaccine effectiveness, 24 Sept 2020
- Three-pronged strategy to a dare-to-innovate culture – 1 Sep 2020
- ARIA-eWMH (for Risk of Cognitive Health) is being offered in the “HSBC Life Eldercare Programme” announced by HSBC Life on 20 June 2019
- Hong Kong SciFest Exhibition 19-22 April 2019
- Pok Oi Hospital Chinese Medicine Culture and Wellness Centre Opening 26 March 2019
- Windows of Health 健康之窗 – A Knowledge Transfer Project of the CUHK
- 香港電台 新䔝荊廣場 – Introduce Biostatistics Research, 29 Jan 2019
- 2018-12-06 大公報: 眼底驗中風,港技術進軍灣區
- Gerontech and Innovation Expo cum Summit, 22-25 November 2018
- Think Global Think Hong Kong, Tokyo Japan – 1 November 2018
- Made-in-HK innovative products help advance management of neurological disorders – MIMS Neurology, 18 May 2018
- HK International Medical Devices and Supplies Fair, 7-9 May 2018
- Health Promotion and Disease Prevention – Economic Daily 2018-3-2
- Guangdong International Applied Science & Technology Expo, 18-20 December 2017
- China (Dongguan) International Science & Technology Cooperation Week, 8-10 December 2017
- Smart Living for Aging Panel Discussion《智能生活渡銀年》研討會
- Hong Kong Biotech Horizon 2017 – The role of big data analytic, machine learning and AI in clinical trials, 22-26 November 2017
- International Alzheimer’s Disease Conference 26-27 May 2017
- Gerontech and Innovation Expo cum Summit (GIES), 16-18 June 2017
- Distinguished Lecture by Professor Heather Cordell, Newcastle University, UK, 24 May 2017
- Chinese Health Information Technology Exchange Conference – Qingdao 18-20 May 2017
- HA Convention 16-17 May 2017
- CUHK Entrepreneur Day 12-13 May 2017
- The Coller Institute of Venture Annual Conference 22-26 April 2017
- 【杏林在線】視網膜血管檢測中風風險 2017-01-09
- 2016 Hong Kong Awards for Industries – Technological Achievement Award
- InnoTech Expo 2016
- International Conference on Innovations in Public Health Sciences
- Asia Pacific Electronic Health Records Conference 2016
- HKTDC Entrepreneur Day 2016
- CUHK Entrepreneur Day 2016
- Hong Kong International Medical Device and Supplies Fair 2016
- Bank of China (HK) “Technology Start-Up” Award (Merit) – 10 Dec 2015
- Hong Kong International Medical Device and Supplies Fair 2015
- International Conference on Biostatistics and Bioinformatics
- Clinical Trials & Biostatistics Shenzhen Research Institute
- 2014 China Big Data International Summit Forum
- Biostatistics and Medical Devices Innovation Symposium
- Seminar by Prof Dongsheng Tu
- ICH GCP Knowledge Test
- Call for application – ACORD 2012
- Critical Appraisal Workshop for Pediatricians
- Social Entrepreneurship in Health Care Sector
- US Biotechnology and Life Science Trade Mission Visit CUHK
- MOU with HiBEAM, Hawii
- The International Symposium on Subjects’ Safety in Clinical Trials
- 1st Asia Pacific Research Ethics Conference
- Seminar by Dr. CY Yeung of MUNIN Corporation
- H1N1 news letter
- Technical Platform for Drug Development(1)
- Technical Platform for Drug Development(2)
- CONTACT US
Sample Size Calculation
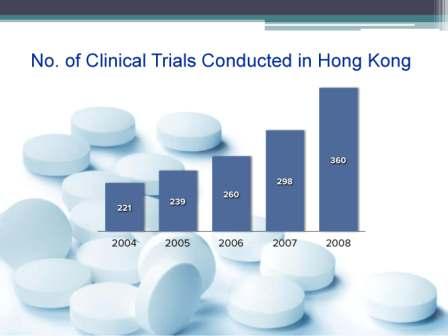
Centre for Clinical Research Biostatistics – Clinical Trials Registry (CCRBCTR)
The Centre for Clinical Research and Biostatistics – Clinical Trials Registry (CCRBCTR), provides all prospective registrants with a convenient means to submit trial registration for all countries. Registered information is displayed in English only (except for the Chinese study title) and is accessible to the public at no charge. Fees would only apply to services other than clinical trials registration, such as translation of registered information. All trial registrations are electronically searchable with an in-house mechanism to ensure the validity of the registration data. With the updated webpage, the registrants can manage all their registrations with one login account and the public can access the audit trail for all registration amendments.
Since December 2009, the Centre for Clinical Research & Biostatistics, Clinical Trials Registry – CUHK is a Partner Registry of a WHO Primary Registry – Chinese Clinical Trials Registry (ChiCTR).We gather trial information and make the information available in the WHO trial registration dataset, so trials registered on our registry are now also registered on ChiCTR with registration numbers from the WHO Primary Registry and all registered information in CCRBCTR is publicly searchable at WHO ICTRP and ChiCTR, Centre for Clinical Research and Biostatistics, Clinical Trials Registry of Chinese University of Hong Kong is now in compliance with WHO ICTRP and ICMJE trial registration requirements and it satisfies the requirements of many major journals.
Clinical Trials Registration Instruction