Help Aids Top
Application: The
determination of the number of patients needed in a prospective comparison of
survival curves in stratified analysis, when the control group patients have
already been followed for some period, following hypotheses with two strata are
usually used: ![]()
where ![]() is the hazard rate of patients in experimental group in
stratum A
is the hazard rate of patients in experimental group in
stratum A
![]() is the hazard rate of patients in control group in stratum A
is the hazard rate of patients in control group in stratum A
![]() is the hazard rate of patients in
experimental group in stratum B
is the hazard rate of patients in
experimental group in stratum B
![]() is the hazard rate of patients in control group in stratum B
is the hazard rate of patients in control group in stratum B
![]() is the weight of
is the weight of![]()
![]() is the weight of
is the weight of![]()
Procedure:
- Enter
a) value of α, the probability of type I error
b) value of β, the probability of type II error, or (1-power) of the test
c) value of K, the proportion of sample in each stratum, the sum of K’s in all strata must be equal to 1
d) value of δ, the minimum hazards ratio, it is specified in alternate hypothesis
e) value of MS, the control median survival (month), it is estimated
from existing control data
f) value of QC, the proportion of patient in control group compared with
experimental group in each stratum
g) value of QE, the proportion of patient in experimental group compared
with control group in each stratum, where QC + QE = 1
h) value of T0, accrual duration (month), the length of time to recruit patients for
study in each stratum
i) value of T-T0, follow-up duration (month, the length of study time of all recruited patients to the end of study T in each stratum
- Click the button “Calculate” to obtain
a) value of N, the sample size of patients in the experimental arm in each stratum
b) value
of DC,
the number of
deaths in control group in each stratum
c) value
of DE, the number of deaths in experimental group in each
stratum
d) value of DT, the
total number of deaths at the end of study in each stratum
e) value of NT, the total sample size for all strata
f) value of NC, the total sample size for control group for all strata
g) value of NE, the total sample size for experimental group for all strata
Assumption:
1.
Time
to survival is exponential distributed with hazard rate λ.
Theory:
In some
cases, the trial may be designed with the intent of conducting a pooled (e.g.,
Mantel-Haenszel) analysis over strata. For simplicity
we consider the case of two strata, say A and B, with sample sizes![]() and
and![]() (
(![]() ), where each stratum may have an associated recruitment and
follow-up period (
), where each stratum may have an associated recruitment and
follow-up period (![]() ) and associated hazards under the alternative hypothesis (
) and associated hazards under the alternative hypothesis (![]() ), For example, strata A and B may correspond to a prognostic
factor (e.g., severity of disease), or stratum A mat refer to a feasibility or pilot
phase and stratum B to the main trial.
), For example, strata A and B may correspond to a prognostic
factor (e.g., severity of disease), or stratum A mat refer to a feasibility or pilot
phase and stratum B to the main trial.
For
each cases, the test statistic would employ pooled estimators of the
within-stratum differences in hazard rated: ![]() , where
, where![]() ,
,![]() are the estimated hazards from stratum A of
are the estimated hazards from stratum A of![]() patients, and likewise for
patients, and likewise for![]() ,
,![]() of
of![]() patients. Further, let
patients. Further, let![]() ,
,![]() be the sample fractions of the two treated groups in stratum
A (
be the sample fractions of the two treated groups in stratum
A (![]() ), and likewise,
), and likewise, ![]() ,
,![]() for stratum B.
for stratum B.
![]()
Under
the assumption of uniform entry with no losses to follow-up,
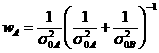 ,
, 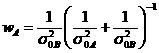
where ![]() ,
, ![]() ,
, ![]()
![]() ,
, ![]() ,
, ![]()
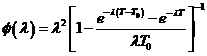
Under the null hypothesis, it then follows that![]()
Under the alternative hypothesis, ![]()
where![]() ,
,![]()
and ![]() ,
,![]()
If we then express the strata samples sizes as fractions of the total,![]() ,
, ![]() , it is easily shown that
, it is easily shown that
![]()
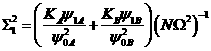
where ![]()
The
basic equation relating sample size and power is
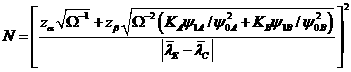
where![]() ,
,![]()
![]() and
and![]() are the strata sample fractions (e.g.,
are the strata sample fractions (e.g.,![]() )
)
Notations:
α: The
probability of type I error (significance level) is the probability of rejecting the true null
hypothesis.
β: The
probability of type II error (1 – power of the test) is the probability of
not rejecting the false null hypothesis.
Example:
Consider the case of two prognostic strata,
such as early-versus late-stage disease, with stratum-specific hazards and
relative strata size KA=0.43
and KB=0.57. Suppose a
clinical trial is to be conducted for a disease with moderate levels of
mortality with hazard rare
It is a two sided test with equal-sized
group (QCA=QEA=QCB=QEB=0.5),
significance level 5% (α =0.05) with
power 80% power (β=0.2), and assume that recruitment was to be terminated after 56 months
of a 124-month study in stratum A (TA=124,
T
Reference:
- Lachin and Foulkes (1986) Biometrics 42: 507-519