Help Aids Top
Application: This procedure is used to test non-inferiority and superiority that can be unified by the following hypotheses:
![]()
When δ>0, the rejection of the null hypothesis indicates the superiority of the test drug over the control.
Help Aids Top
Application: This procedure is used to test non-inferiority and superiority that can be unified by the following hypotheses:
![]()
When δ>0, the rejection of the null hypothesis indicates the superiority of the test drug over the control.
When δ<0, the rejection of the null hypothesis indicates the non-inferiority of the test drug against the control.
Procedure:
a) value of α, the probability of type I error
b) value of β, the probability of type II error
c)
value of allowable difference
d) value of Population variance
e) value of δ>0, the superiority margin or value of δ<0, the non-inferiority margin.
Formula:
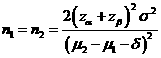 (*)
(*)
Notations:
α: The
probability of type I error (significance level) is the probability of rejecting the true null
hypothesis.
β: The
probability of type II error (1 – power of the test) is the probability of
failing to reject the false null hypothesis.
δ: Clinically meaningful difference. The largest change from the reference value (baseline) that is considered to be trivial.
μ2 – μ1: Value of allowable difference is the true mean difference between a test drug (μ2) and a placebo control or active control agent (μ1).
Examples
Example 1: Consider the difference of 5% is a difference of clinical importance, thus the non-inferiority margin is chosen to be 5% (i.e., δ=-0.05). Also, suppose the true difference in mean of low density lipidproteins (LDLs) between treatment groups is 0% (i.e., μ2(test) – μ1(control)=0). Thus, by using (*), with the standard deviation is 10% (i.e., expected variance is 0.01), the required sample size to achieve an 80% power (β=0.2) at α=0.05 for correctly detecting such difference of 0.5 change obtained by normal approximation as n=50.
Reference: Chow, Shao and Wang, Sample Size Calculations In Clinical
Research,