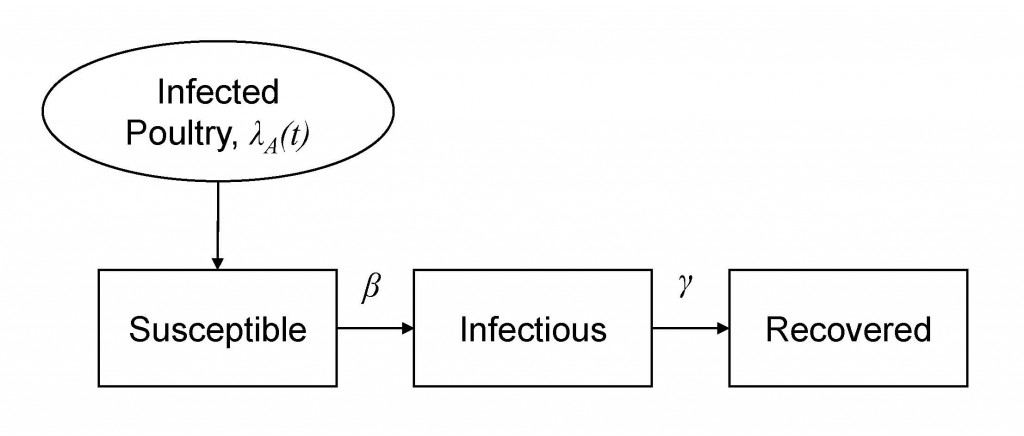
We develop mathematical models for describing the behaviour of a system in different public health areas. Mathematical models can take many forms, including but not limited to, statistical models and system dynamics models, etc. They are instrumental in health policy evaluation and modelling of infectious diseases transmissions but also in assessment intervention effects in clinical trials.
Infectious Disease Modelling
Owing to ethical challenges in many experimental studies (e.g. travel restriction in influenza A/H1N1 pandemic), mathematical models provide an important tool to assess the intervention effectiveness by using computational simulations. Over the past years, the tradition susceptible-infectious-recovered (SIR) models and its extension have been applied to several infectious diseases such as influenza, measles, and tuberculosis. DBIO biostatisticians collaborate with public health officials such as Zhejiang and Hubei Provincial Center for Disease Control and Prevention (CDC) on studies of evaluation of interventions on epidemic controls.
Health Policy Modelling
Mathematical models for health policy are well suited to address dynamic complexity problems in public health. Through employing the models, policy options with the potential of improving health can be built-in and tested in various scenarios and combinations with stakeholders such as health practitioners, health economists, and decision-makers. DBIO works with different local officials such as Hospital Authority, Food and Health Bureau on hospital resources allocations and elderly care management.
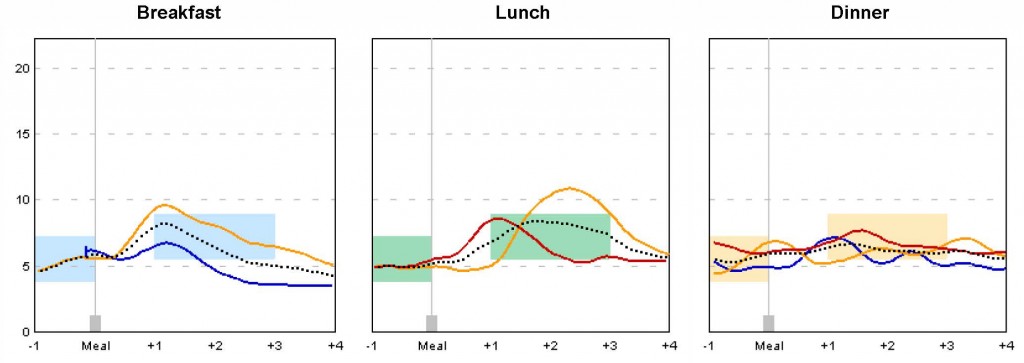
Clinical Trials Modelling
Statistical models are useful in planning clinical trials and decision making, especially for those in initial phases with limited data. DBIO has many academic and industrial partners in clinical trial studies. A recent investigation on the use of mathematical models in Continuous Glucose Monitoring Systems (CGMS) data can greatly enhance the study power of a proof-of-concept trial for patients with pre-diabetes.