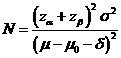Help Aids Top
Application:
This procedure is used when the test object (i.e.,
drug) is concluded to be equivalent to a gold standard on average under an
acceptable superiority margin. The following hypotheses are usually considered: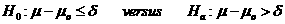 .
.
When δ>0,
the rejection of the null hypothesis indicates superiority over the reference value.
When δ<0, the rejection of the null hypothesis implies non-inferiority against the
reference value.
Procedure:
- Enter
a)
value
of α,
the probability of type I error
b)
value
of β,
the probability of type II error
c)
value of allowable difference
d) value of population
variance
e)
value
of δ>0,
the superiority margin or value of δ<0, the non-inferiority margin.
- Click the button “Calculate” to obtain result sample size N.
Formula:
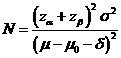 (*)
(*)
Notations:
α: The
probability of type I error (significance level) is the probability of rejecting the true null
hypothesis.
β: The
probability of type II error (1 – power of the test) is the probability of
failing to reject the false null hypothesis.
δ: Clinically meaningful difference. The largest change from the reference value (baseline) that is considered to be trivial.
μ – μ0:
The value of allowable difference is the difference
value between true mean and reference mean (constant value).
Examples
Example 1: A pharmaceutical company
is studying for prevention of progression from osteopenia to osteoporosis they wish to show that the mean
bone density post-treatment is no less than pre-treatment by a clinically
meaningful (i.e., reduce AMBD) difference δ=-0.5 to test non-inferiority. The difference of mean bone density between
before and after treatment is expected to be μ–μ0=0.5. Then
the result of sample size at α=0.05 with population variance of 1
for having β=0.2 is N≈7.
Reference: Chow, Shao and Wang, Sample Size Calculations In
Clinical Research, Taylor & Francis, NY.
(2003) Pages 50-52.
Top
![]() .
.